Stuck like superglue
Scientists have developed a promising new method of fixing biological molecules to each other
Scientists at the University of Oxford have developed a pair of protein fragments dubbed ‘SpyTag’ and ‘SpyCatcher’ that form an incredibly strong bond. Within minutes of being brought into contact the protein fragments form a covalent bond so strong that when put under pressure, the protein breaks away from the apparatus before the bond breaks. It is effectively a biomolecular superglue – except, due to its good specificity, without the possibility of accidentally sticking to the edge of the test tube or other unwanted molecules.
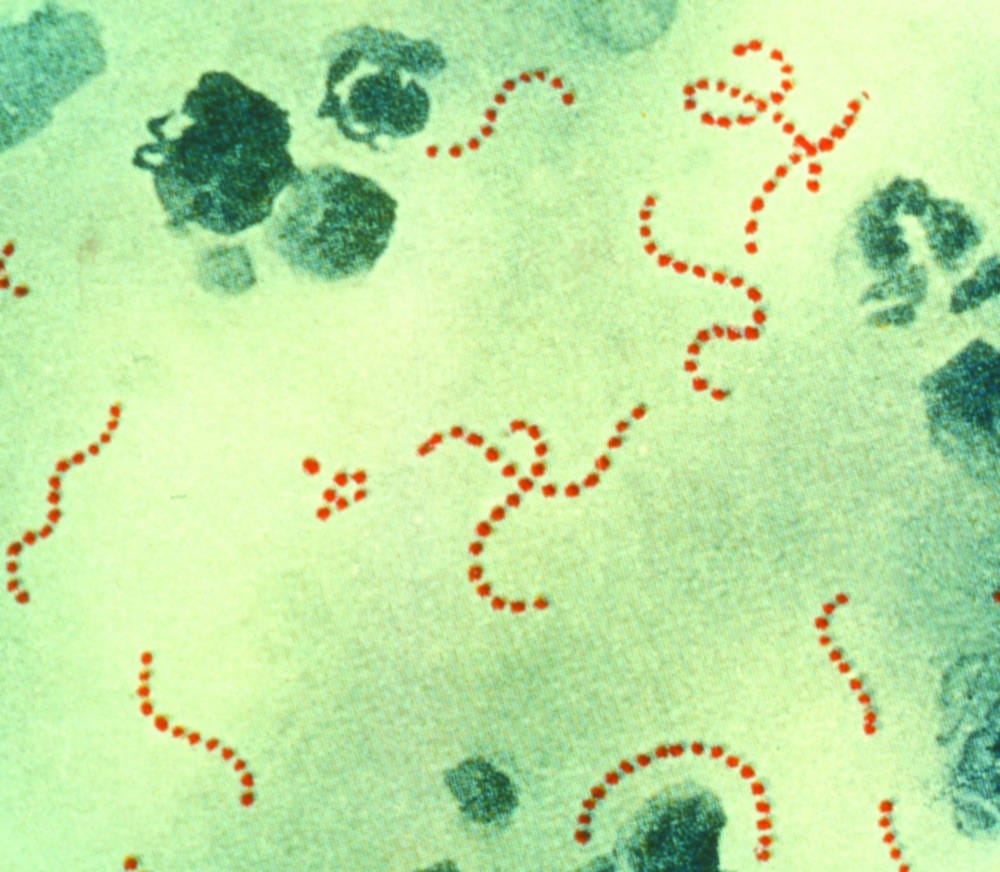
The discovery was inspired by Streptococcus pyrogenes, which causes infections ranging in severity from sore throats to toxic shock syndrome. The bacterium uses the FbaB protein to bind to human cells before invading. The particular strength of this link is caused by the protein’s shape. Rather than simply being made of a chain of covalently bonded amino acids held together by comparatively weak forces, FbaB forms amino acid loops which are subsequently held together by an additional covalent bond. The team of scientists reasoned that, if they were able to successfully engineer the protein in such a way as to allow the creation of two separate fragments which would form this covalent bond again once brought together, the same mechanism could be used to attach other molecules to each other.
The bond is not only very selective, forming between SpyCatcher and SpyTag rather than any other molecule in the cell environment, but it also forms in a large range of conditions. The team successfully repeated the experiment not only at 37°C and neutral pH, mimicking a mammalian cell, but also at 4°C in high concentrations of detergent and under acidic conditions. This was true not only in vitro but also when tested inside Escherichia coli bacteria.
This development is a leap forward towards the goal of being able to combine different proteins at will and with ease. Unlike previously discovered non-covalent equivalents, which can be broken by sufficient shear forces within milliseconds, the SpyCatcher:SpyTag complex cannot be broken by molecular motors. Other existing approaches binding biomolecules using covalent bonds are similarly strong but slow, associated with poor yields, or in need of reaction conditions hostile to common life forms.
Unfortunately, unlike other mechanisms, SpyTag is not traceless. Also, the rate of bond formation between SpyTag and SpyCatcher is too slow to be effective inside living cells; diffusion is likely to occur before bonding. With further research and optimization, however, we may soon see the dream of biological Lego come true.