Xenon could be used to reduce traumatic brain damage, Imperial study finds

Traumatic Brain Injury (TBI) is the leading cause of death and disability for people aged under 45 in developed countries. The first ever life-long study in mice has found that head injury effects can be halted by xenon gas. Following TBI, xenon prevented the mice from early deaths, improved their long-term cognition, and protected their brain tissue.
In TBI, a primary injury is first caused by the initial force from a fall or car accident, for example, and is followed by a secondary injury which develops in the minutes, hours and days afterwards. It is the secondary injury that is largely responsible for the mental and physical disabilities associated with TBI - but there are currently no specific drug treatments that can be given after the trauma to stop TBI from developing. The only treatment available is supportive and rehabilitative. TBI patients who survive the injury have a reduced life expectancy and an increased risk of developing Alzheimer’s disease or other dementias later in life.
Now, researchers from Imperial College London and Johannes Gutenberg University Mainz have found that the anaesthetic drug xenon, given shortly after a TBI, prevents early death and long-term cognitive impairment and even protects the brain tissue itself in mice. The xenon-treated mice had similar life expectancy, cognitive function, and brain tissue integrity to mice that had never sustained a TBI.
Previously, the same team led by Dr Robert Dickinson and colleagues at Imperial’s Department of Surgery and Cancer, showed that xenon limited early brain damage and improved long-term motor function in mice with TBI, but longer term effects were not yet investigated. Now for the first time, the effects of xenon over the whole lifespan of mice has been observed, in this study that was published in the British Journal of Anaesthesia.
Animals were randomly allocated to one of three groups: TBI xenon, TBI control, and healthy control. Under general anaesthesia and long-acting pain relief, a controlled mechanical force was applied to the brains of the TBI control and TBI xenon groups. The healthy control group was given anaesthesia, but did not receive a TBI. The researchers gave xenon gas to one of these groups (TBI xenon group), while the other two received control gas for the same amount of time.
All three groups then underwent learning and memory tests at two weeks and 20 months after the injury. Time of death was recorded and the mice brain tissues were examined.
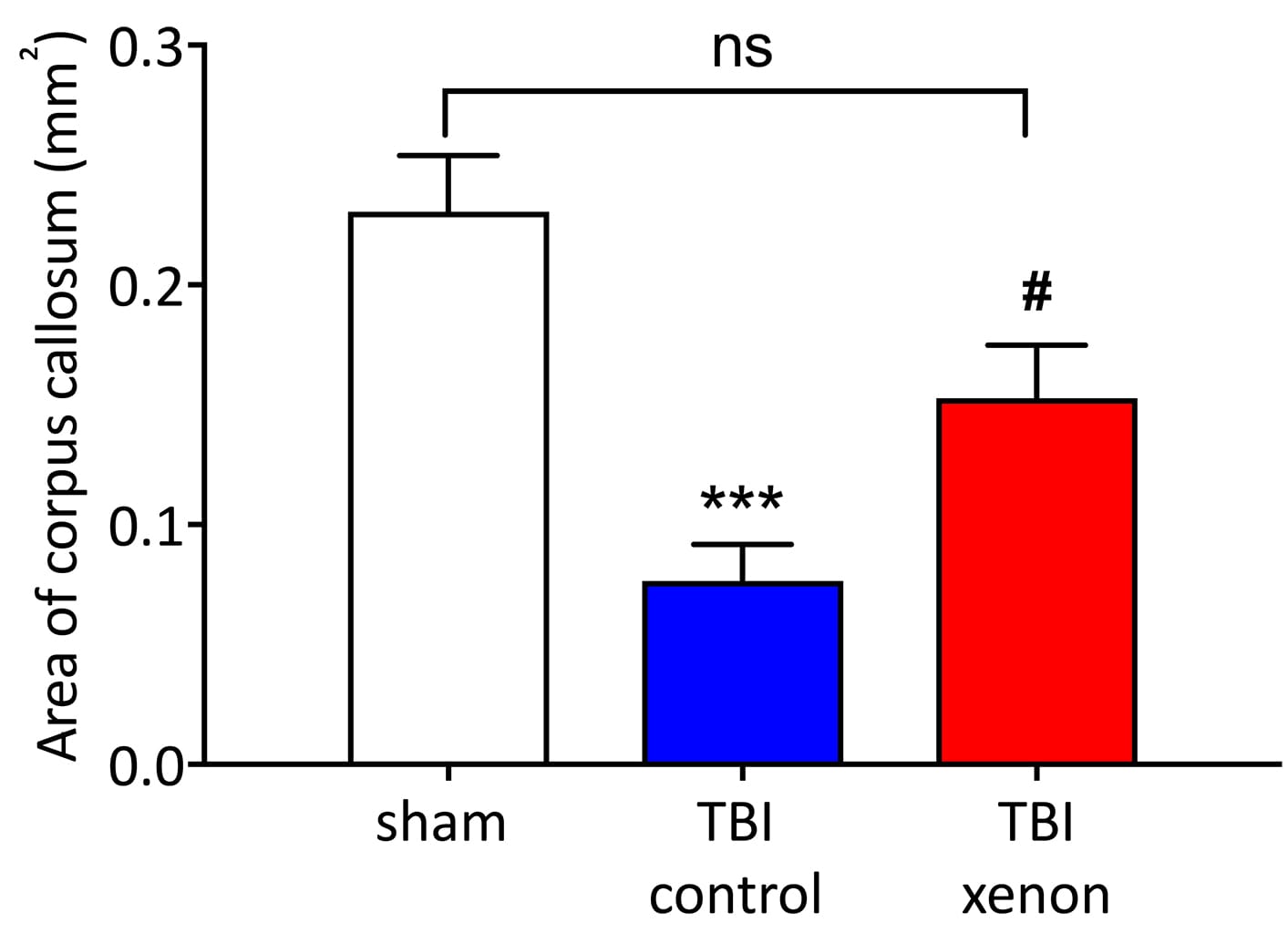
The researchers found that the TBI xenon group had the same life expectancy as the healthy control group which had not suffered a TBI. However, the TBI control group developed late-onset cognitive damage. Xenon treatment shortly after TBI appeared to prevent this. The TBI control group were also observed to have damage in key brain areas involved in cognitive functioning. Xenon-treatment prevented or significantly reduced this damage.
Xenon prevented the loss of brain cells in the hippocampus (an area of the brain associated with learning and memory), and prevented degeneration of nerve fibres in the corpus callosum (which connects the two brain hemispheres). Xenon was also shown to reduce long-term brain inflammation that is believed to be involved in cognitive impairment in Alzheimer’s Disease and other dementias.
According to the group, these findings are important as they could offer insight into new treatments for TBI patients, who currently do not have any specific drug treatments available.
Lead author of the study, Dr Rita Campos-Pires, from the Department of Surgery & Cancer, said, “There is currently a huge gap in what treatment we can offer to patients who’ve suffered TBI - an injury which can impact all areas of their lives. Although xenon has not yet been tested for TBI in humans, our findings add to the growing body of evidence that suggests it could be used after head injuries to prevent secondary injury developing.
“Xenon appears to act in a variety of ways, but one of the most likely mechanisms to explain its protective effects on brain tissue is by inhibiting receptors in the brain known as NMDA receptors, that become over-activated following a brain injury.”
Dr Dickinson added: “We have looked at very long-term outcomes, up to 20 months after TBI in mice. This is very rarely done in animal studies and is equivalent to following up human TBI patients until their 80s. The finding that only a short treatment with xenon can have beneficial effects on cognition, survival, and brain damage almost two years later suggests that xenon might in future prevent cognitive decline and improve survival in human TBI patients.”
Xenon is already used as a human general anaesthetic, is known to have few side effects and could be easily given via inhalation or to mechanically ventilated TBI patients in the intensive care unit.
Given xenon’s safety profile - and today’s findings - the researchers hope in the future to evaluate the effectiveness of xenon in human TBI patients.