Genetically modified pig kidneys transplanted into brain-dead patient
Could this be the answer to organ shortages?
For the first time in the history of medicine, two genetically engineered pig kidneys were transplanted into a man who was brain-dead at the University of Alabama (UAB) in Birmingham. The recipient Jim Parsons, 57, had been declared officially dead following a severe head injury during a motorcycle race. With his family’s blessing, Jim was kept alive on a ventilator so that researchers could perform the experiment just four days later. With his blood still circulating, his own kidneys were removed, and replaced with the genetically engineered pig kidneys.
Why can we not use ordinary animal organs?
On average, 20 people die every day on the transplant waiting list. However, under normal conditions, pig organs cannot be transplanted directly into humans. There are three main obstacles preventing pig-to-human transplants (xenotransplantation):
(1) Risk of rejection by the immune system
(2) Potential transmission of cross- species infections
(3) Ethical concerns.
This is why the historic experiment on Jim Parsons is “a major milestone in the field of xenotransplantation, which is arguably the best solution to the organ shortage crisis,” according to lead surgeon Dr Jayme Locke, director of the Comprehensive Transplant Institute at the UAB. “We have bridged critical knowledge gaps and obtained the safety and feasibility data necessary to begin a clinical trial in living humans with end- stage kidney failure disease.”
What makes this experiment different?
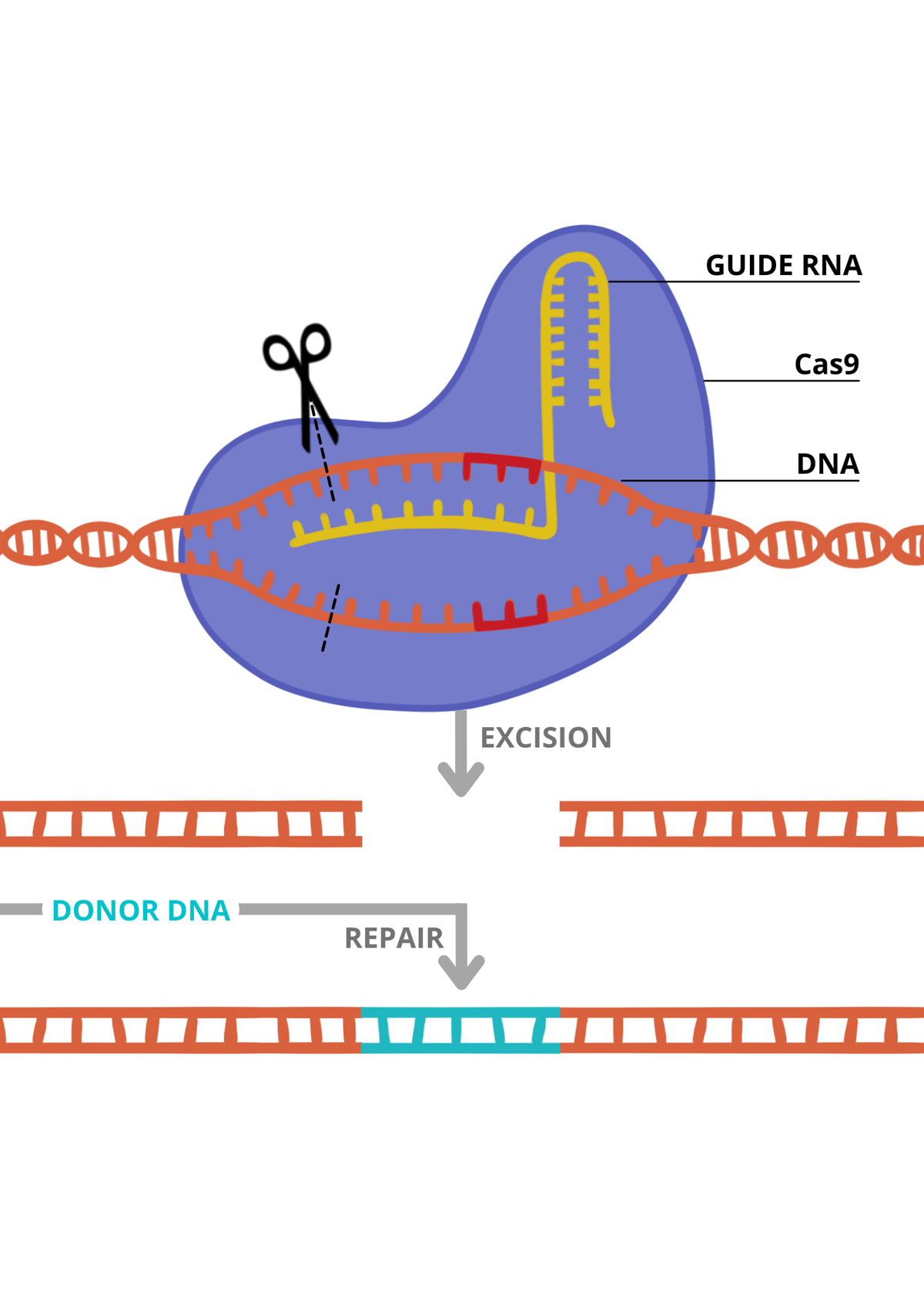
Locke’s team is the first to test therapies in brain dead people. The experiment relating to Jim Parsons, now termed the Parsons model, was developed to test the safety and feasibility of using pig organs in preparation for clinical trials. This could be an alternative when testing on animals is insufficient. Normally, pig organs are too different to human organs, so they cannot be transplanted as they are rejected by the human immune system even if people are taking drugs to suppress their immune system. However, new pigs have been genetically modified by the US firm Revivicor and designed to prevent rejection, using CRISPR, a gene-editing technique (see information panel).
The pig kidneys provided by Revivicor came from a 13-month-old male pig and had under gone 10 gene edits by CRISPR, which switched off four genes, including genes involved in rejection by the human immune system. Additionally, the pig’s genes had six human genes added.
Before transplant, the cross-matching process took place. This was where the blood from the donor pig and Mr Parsons were mixed together to ensure that, when transplanted, his immune cells would not attack the pig’s cells. The new pig kidneys were then transplanted into Mr Parsons, using the same connections as his original kidneys. During the 77- hour experiment, the pig kidneys were not rejected, and within 23 minutes the first kidney to be transplanted began to produce urine. “It’s a remarkable achievement. We had a beautiful pink kidney, not one that turned black from hyperacute rejection,” said Dr Locke. The experiment was stopped once Mr Parsons could not be maintained on mechanical support any longer.
Conclusions
Overall, the surgeons concluded that the experiment was a success and it shows potential for progression into clinical trials. “The goal is not to only help one person, but to help everybody,” said Dr Locke.
Despite these positive results, getting genetically modified pig organs is still extremely difficult. Some scientists believe experiments should focus on clinical trials, as opposed to experiments on brain-dead patients. “What we really want to know is will the pig kidneys function for a year,” said Dr Cooper, from Massachusetts General. He believes the only way to truly see if using animal organs is a potential solution is to transplant organs into living patients. However, the Parsons experiment may allow for FDA approval for clinical trials and is a historic step forward in the field of transplantation.
“A radical solution is needed for the organ supply crisis”, said Dr Locke.









