Nobel Prize in Medicine 2025: The Guardians of Tolerance
Mary E. Brunkow, Fred Ramsdell and Shimon Sakaguchi were awarded the prize "for their discoveries concerning peripheral immune tolerance"
A hallmark of the adaptive immune system is its ability to recognise pathogens the body has never encountered before. This versatility is driven by B and T cells, which detect antigens (molecular signatures) and mount a tailored immune response.
It is as if our immune system is forging a key for every possible lock it might face. This vast diversity of “keys”, known as antigen receptors, arises from genetic recombination, allowing it to recognise virtually any pathogen (even a futuristic space virus from a sci-fi movie!)
But producing every possible key comes with a risk: inevitably, some of these keys will fit our own “locks”. When this happens, the immune system may recognise and attack the body itself, leading to autoimmune diseases.
To prevent this, our immune system must learn to recognise our tissues without attacking them. For decades, scientists believed this self-tolerance was only achieved through a process called central tolerance, a selection process in the thymus or, as scientists like to call it, the “murder university”. Developing cells undergo rigorous tests, and only those who do not react to self, are able to survive, graduate and enter our blood circulation (they do not get to go to Royal Albert Hall like us…).
After the discovery of central tolerance, some researchers suspected some self-reactive cells might somehow “escape” the thymus. However, this idea had no solid foundations and was quickly dismissed. Still, in the 1980/90s, Japanese scientist Professor Shimon Sakaguchi, working in Nagoya decided to challenge the rules.
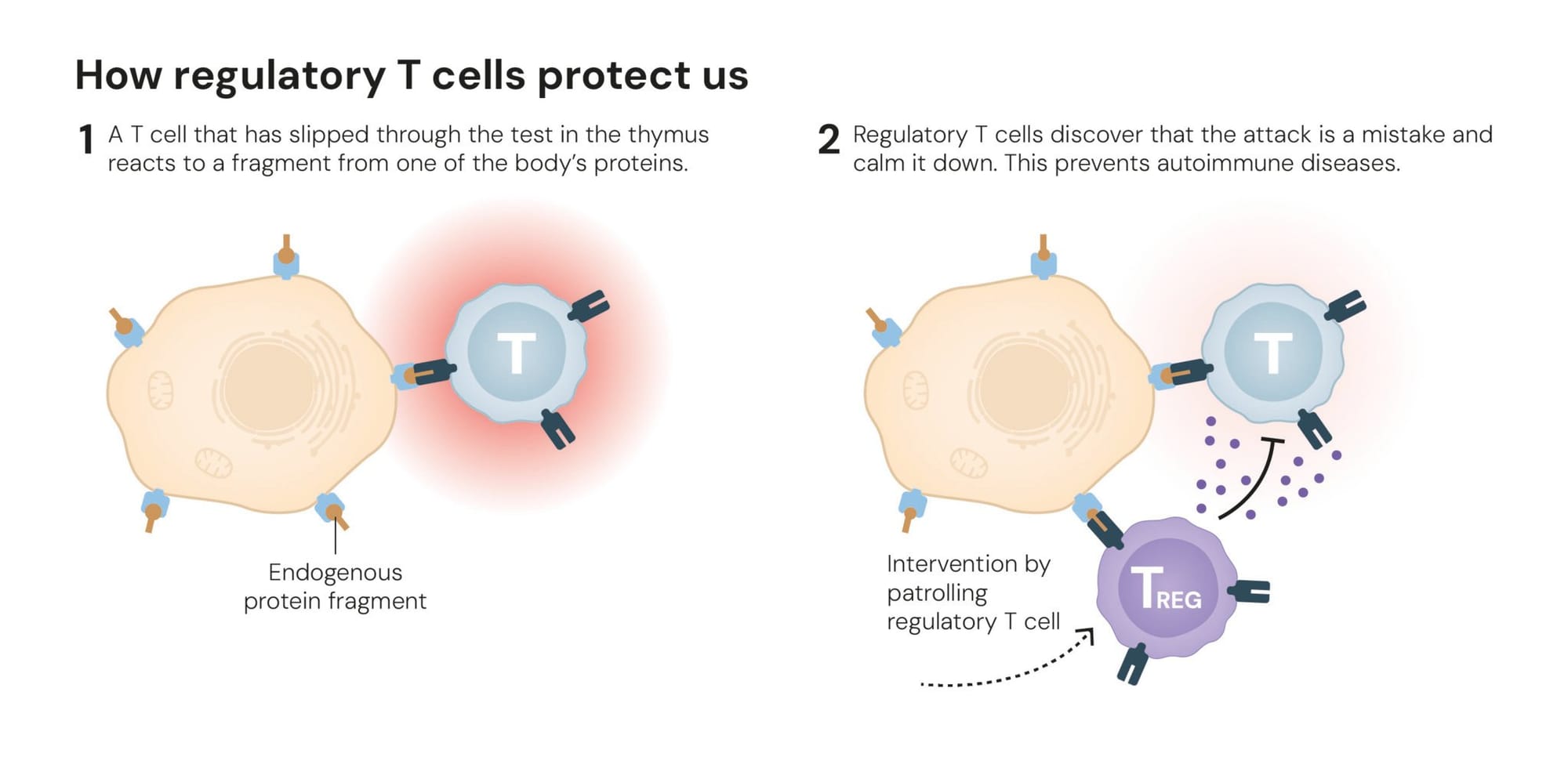
He found that removing the thymus from newborn mice (therefore allowing self-reactive cells to be formed) led to fatal autoimmunity, but transferring mature T cells from healthy mice donors prevented it!
This suggested the existence of “guardian angels” cells preventing the activity of an auto-reactive immune system. He later identified them as CD4+T cells expressing CD25 and named them regulatory T cells (Tregs). In his groundbreaking paper, he stated “accumulating experimental evidence now suggests that removal or inactivation of a certain regulatory T cell population can break natural self-tolerance, leading to spontaneous development of various autoimmune diseases”.
Meanwhile, in Tennessee, researchers studying the effects of radiation stumbled across a peculiar mouse strain with scaley skin, enlarged spleen, swollen lymph glands, and a short lifespan. These “scurfy” mice sparked the curiosity of our Nobel Laureates, Dr. Mary E. Brunkow and Dr. Fred Ramsdell who realised the defect was associated with the X chromosome, therefore explaining why it affects mostly male mice. Years later, they identified the reason, a mutation in the FOXP3 gene. Remarkably, the same mutation was found in humans with a rare autoimmune disorder called IPEX, mostly affecting people with XY chromosomes. This revealed that FOXP3 is essential for immune tolerance in humans too!
Thanks to scientific collaboration and data-sharing, the puzzle pieces finally fit: the Tregs found by Sakaguchi were governed exactly by the master regulator gene FOXP3.
This process, known as peripheral tolerance, occurs when self-reactive T cells leave the thymus but, instead of promoting autoimmunity, they downregulate autoimmune activity and promote balance.
Since this discovery, we’ve learned that Tregs not only prevent autoimmunity but also calm the immune system down after an infection, a key step to prevent tissue damage and restore balance.
We had the immense pleasure of speaking with Professor Marina Botto, Head of the Department of Immunology and Inflammation, who reflected:
“These discoveries not only deepened our understanding of immune regulation but also laid the groundwork for therapeutic strategies targeting autoimmune diseases, cancer, and transplant rejection.
In an era where immunotherapies are reshaping medicine, this recognition underscores the importance of immunological research”.
The list of Treg-based therapies has grown rapidly in recent years and will not stop soon. Future research may include engineered therapeutic Tregs, exploring their roles in neuro-degeneration, aging or metabolic diseases, and how external factories such as diet or the microbiome shape their function.
Beyond medicine, this Nobel prize teaches us something timeless: Don’t be afraid to swim against the tide, trust curiosity, and don’t forget to share your science – it may just give you the next Nobel Prize!